ProBio LVV Manufacturing
ProBio is experienced in manufacturing lentiviral vectors adopting both adherent system and suspension system. We use licensed PowerATM-293T cell lines, and developed our proprietary suspension cell line PowerSTM-293T, with the production capacity from 2L to 200L.
Our experts will discuss your project with you in detail and propose a best solution to accelerate your project.
Suspension Culture System
- Proprietary cell line PowerSTM-293T
- Stable and scalable suspension manufacturing process
- up to 200L manufacturing scale
- 4~5 times final yield improvement comparing with adherent culture system
- ~50% cost reduction
- High T cell transduction efficiency
PowerSTM-293T
- Proprietary Suspension cell line
- License for IND, clinical and commercial
- Royalty free, maintenance free
- Excellent performance in producing lentiviral vector
LVV Production: PowerSTM-293T with Different GOI
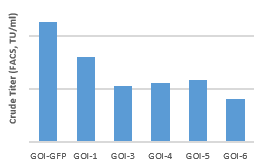
Crude titer up to 108 TU/ml
Adherent Culture System
- The largest manufacturing scales is Up to 96L
- Closed culture system reduces contamination and improves stability
- Full-automatic operation improves reproducibility and stability
Accumulated Experience
ProBio has accumulated rich experience in manufacturing lentiviral vector, which guaranteed a dedicated and professional service for customers.
- Worked with global clients for their LVV projects
- Provided GMP lentiviral vector for CAR-T, TCR-T projects
- Over 100 GMP manufacturing batches experience



